Vaxxon® SRP® Pasteurella
A NEW BREED OF CHOLERA VACCINES
Vaxxon® SRP® Pasteurella
FINALLY, A VACCINE TECHNOLOGY WHICH FUNCTIONS INDEPENDENTLY OF SEROTYPE
Vaxxon® SRP® Pasteurella Bacterial Extract Vaccine is a cholera vaccine using conserved siderophore receptor and porin (SRP) proteins as immunogens and manufactured using proprietary SRP Technology developed by Vaxxinova US.
An innovative option for Cholera control in layer and broiler breeder chickens.
EFFECTIVE:
Protects against mortality from fowl cholera caused by
P. multocida serotype 1 (the only USDA approved challenge strain in chickens)
- Improved livability in affected flocks
INNOVATIVE:
Utilizes bacterial surface proteins that are not serotype specific
- Vaccine strains were selected to broadly represent the SRP proteins commonly found among many P. multocida serotypes
- Proteins (including SRP) are known to produce strong anamnestic response
SAFE:
No adverse events observed in field safety studies following vaccination
- Removal of LPS (endotoxin) reduces systemic reactivity compared to bacterins
- Faster resolution of local injection site lesions compared to bacterins

A brief history of Fowl Cholera
Fowl Cholera (FC) is a contagious acute septicemic disease that is economically important in intensive poultry operations due to the high morbidity and mortality of flocks. The infection, caused by Pasteurella multocida, can lead to chronic infection in the flock resulting in birds with swollen hocks and wing joints.1,2
In addition to sanitation, good farm management, and strict biosecurity, vaccines are important in preventing fowl cholera. Live vaccines provide good protection against multiple serotypes but can cause mortality or subclinical disease in the vaccinated birds. Inactivated whole cell bacterins requires matching the genotype and lipopolysaccharide (LPS) structure to the field challenge strain to be effective.
SRP vaccines induce antibodies that block bacterial siderophore receptors preventing entry of critical iron molecules leading to death of the bacteria.
EFFICACY:
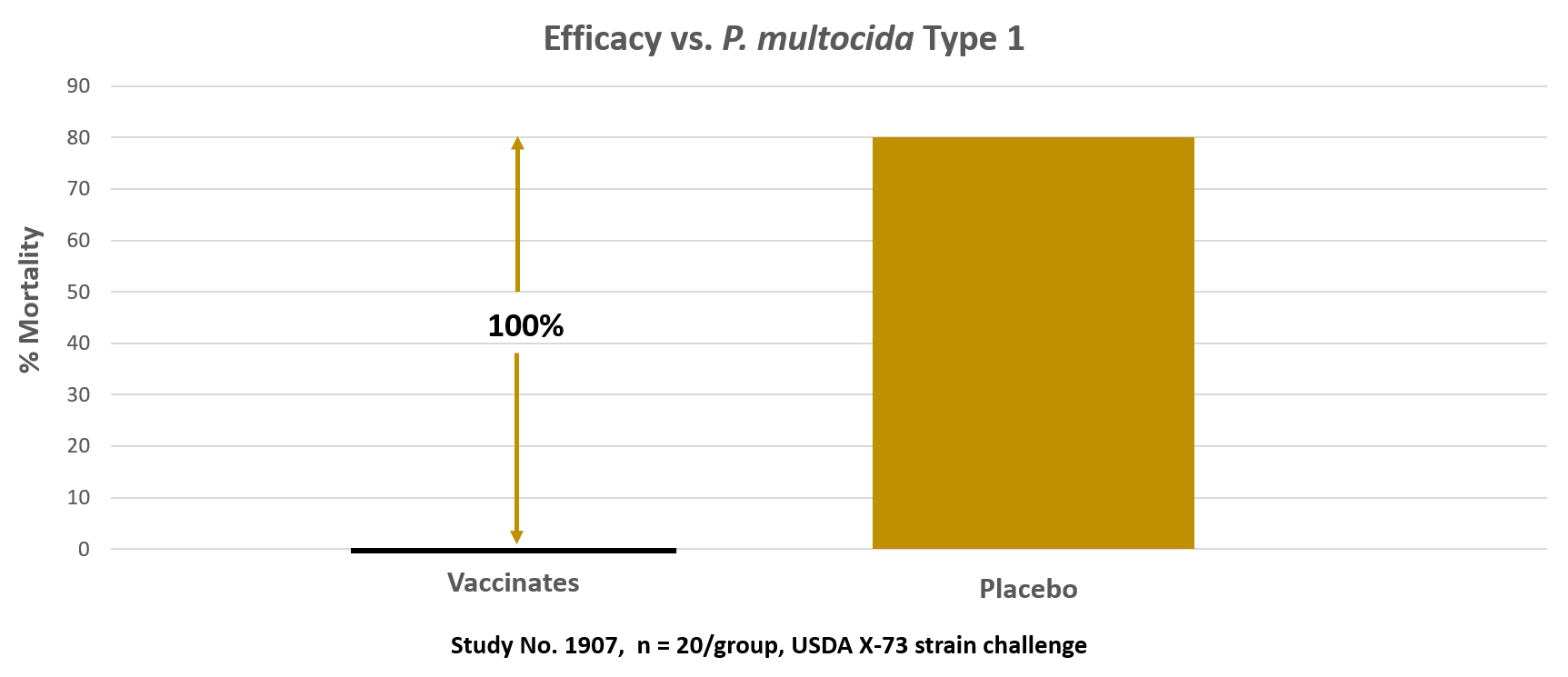
This graph shows the % mortality at 14-days post-challenge with P. multocida Type 1 (using the USDA X-73 challenge strain). There were no mortalities or 0% of the Vaxxon SRP Pasteurella vaccinated group died during the 14-day observation period. 80% of the placebo/non-vaccinated birds died after challenge. This is equivalent to a 100% efficacy based on Prevented Fraction analysis. The challenge strain was isolated in the livers of all dead birds.
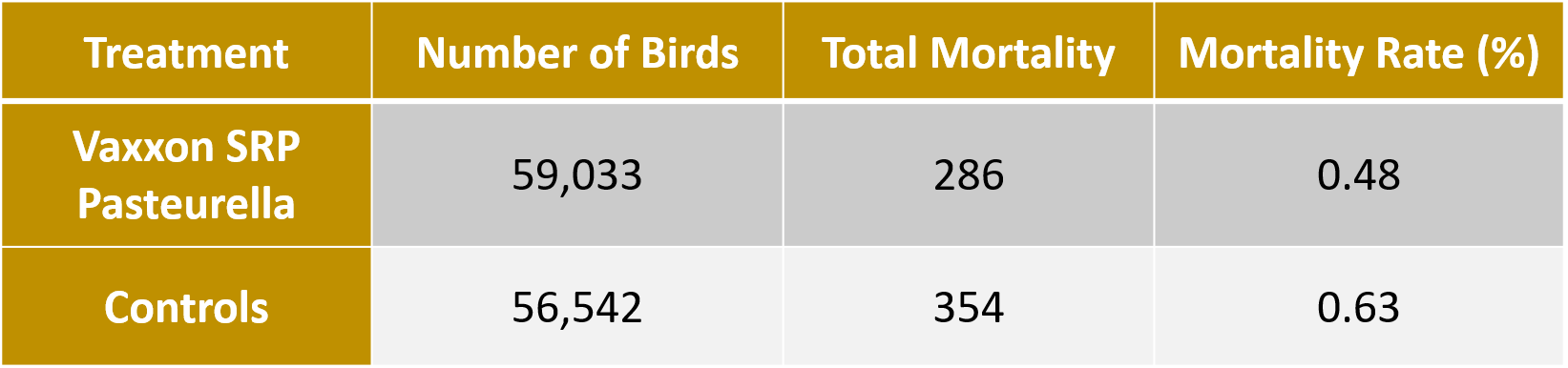
Field Safety Mortality Data
This table shows the number of birds administered the Vaxxon SRP Pasteurella and control (bacterin) vaccines, the number of birds that died in those flocks and the equivalent mortality rates. Birds administered the Vaxxon SRP Pasteurella vaccine had numerically less birds dying during the observation period compared to the control group.
POULTRY RESOURCES




Worthington office
1520 Prairie Drive
Worthington, MN 56187
(800) 220-2522
info.us@vaxxinova.com
Willmar office
1801 Biotech Ave NE
Willmar, MN 56201
(844) 777-8299 (SRP-VAXX)
info.us@vaxxinova.com
Copyright © 2024. All rights reserved.