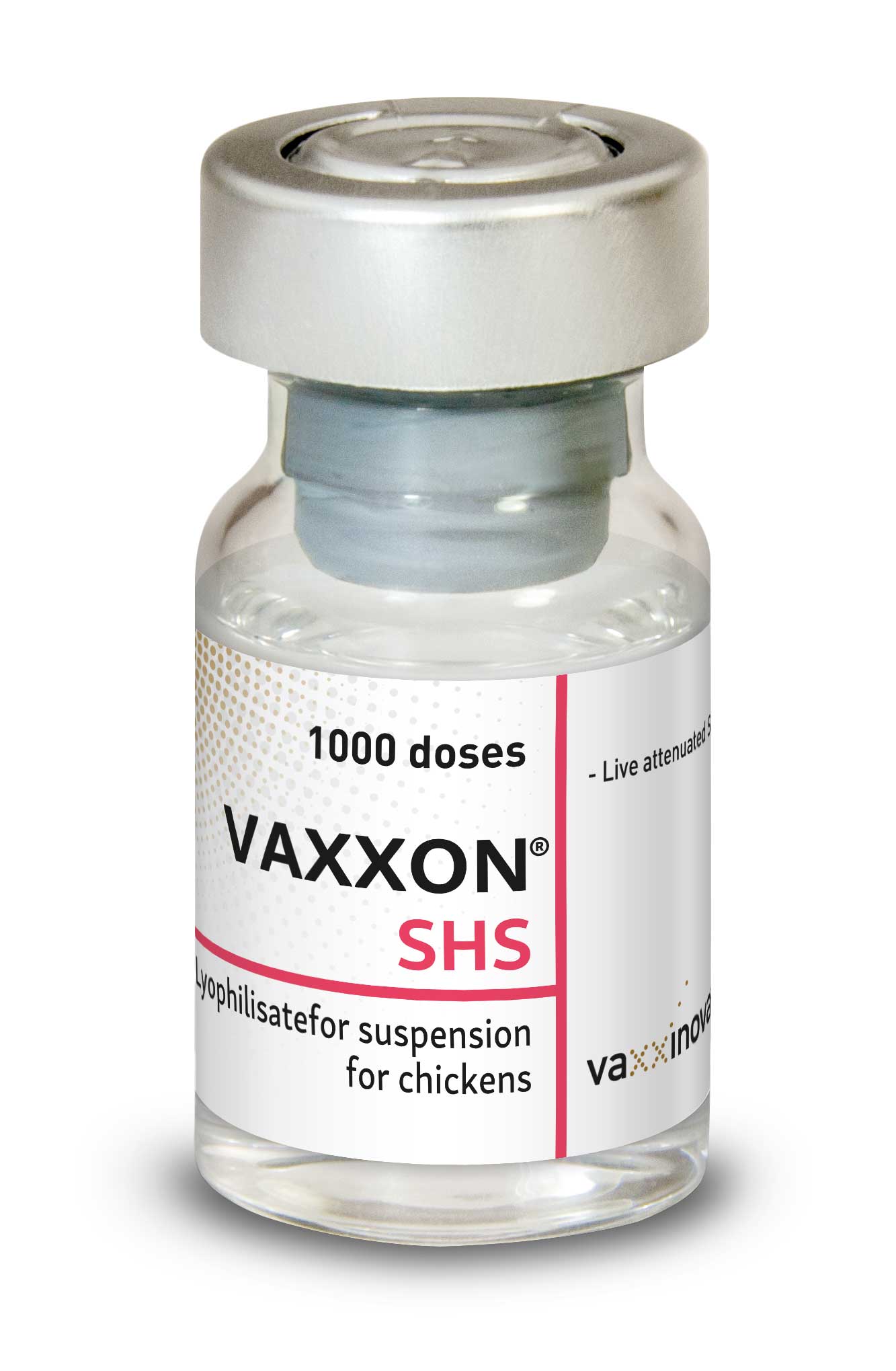
Vaxxon® SHS
Swollen head syndrome, or metapneumovirus, live vaccine now available
Vaxxon® SHS
USDA Approves First-Ever Import of Live Vaccine into the United States
Willmar, Minnesota — Vaxxinova US proudly announces a groundbreaking development for animal health and the poultry industry. The United States Department of Agriculture (USDA) has approved importation of a live Avian metapneumovirus vaccine, Vaxxon® SHS, into the U.S. market. This unprecedented approval marks a significant milestone in the fight against Avian metapneumovirus (aMPV).
Vaxxon® SHS is a lyophilized live attenuated metapneumovirus vaccine to protect against Swollen Head Syndrome in poultry. Developed by Vaxxinova Italy, this innovative vaccine has demonstrated exceptional efficacy and safety through rigorous testing and evaluation.
“This landmark approval is a testament to our commitment to advance animal health and meet the needs of our customers” said Brian Harberts, Managing Director, spokesperson for Vaxxinova. “The USDA’s decision marks an important milestone for the US turkey and poultry industry who have been devasted by aMPV.” “As a result of the collaboration with industry organizations such as National Turkey Federation, poultry producers, and the USDA, Vaxxinova US is now able to offer the same vaccine to the US market that Vaxxinova Italy has been providing to customers for the past twenty plus years”.
“This landmark approval is a testament to our commitment to advance animal health and meet the needs of our customers”
Dr. Dan Domingo, Senior Poultry Technical Service Veterinarian at Vaxxinova, states that “In addition to good biosecurity, the use of live attenuated virus vaccines followed by administration of inactivated (killed) virus vaccines have been successfully used to control Avian Metapneumovirus, especially in long-lived chickens and turkeys. Live vaccines have the ability to stimulate both systemic and local immunity in the respiratory tract.”
Additionally, “Attenuated live virus vaccines for aMPV prime the birds for the effective use of inactivated vaccines.”
Vaxxinova is honored to collaborate with the USDA, and our international Vaxxinova colleagues around the world, and other key stakeholders to bring this innovative solution to the U.S. market. Distribution of Vaxxon® SHS Live vaccine is expected to commence in early 2025, first focusing on the needs of the turkey industry. Additionally, Vaxxinova will have an inactivated autogenous aMPV vaccine available for US producers to provide the Live vaccine priming and Inactivated boost the industry has been hoping to have available.
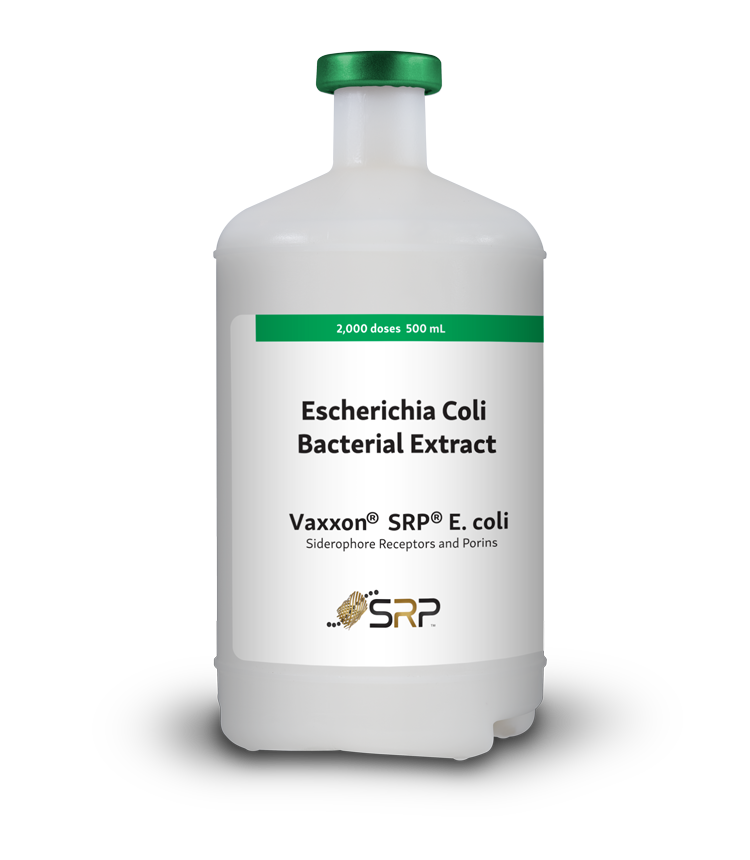
Experimental Autogenous aMPV vaccine
USDA allows the sale of inactivated (killed) avian metapneumovirus vaccine
Vaxxinova currently sells an autogenous experimental metapneumovirus vaccine, sub-type A. Contact us to learn more.
Get started today!
POULTRY RESOURCES



Worthington office
1520 Prairie Drive
Worthington, MN 56187
(800) 220-2522
info.us@vaxxinova.com
Willmar office
1801 Biotech Ave NE
Willmar, MN 56201
(844) 777-8299 (SRP-VAXX)
info.us@vaxxinova.com
Copyright © 2025. All rights reserved.